Activation energy endothermic vs exothermic reactions
Data: 27.09.2017 / Rating: 4.8 / Views: 929Gallery of Video:
Gallery of Images:
Activation energy endothermic vs exothermic reactions
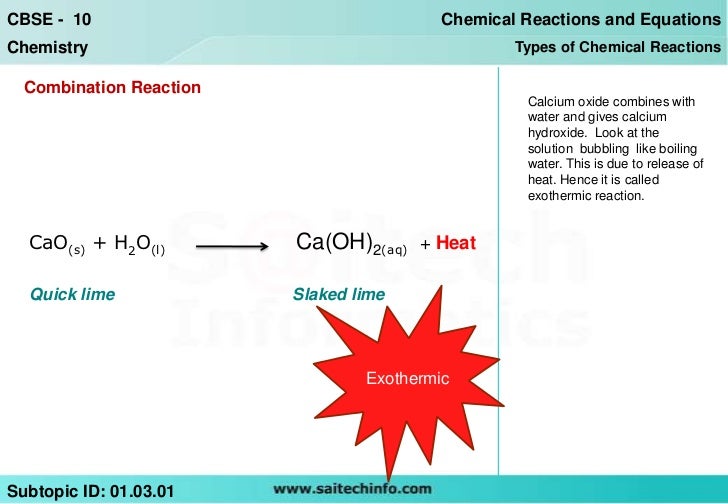
Can the activation energy of a catalytic reaction have a (which is exothermic) If we need to compare two reactions with activation energy 40kJmol. What's the difference between Endothermic and Exothermic? An endothermic reaction occurs when energy is absorbed from the surroundings in the form of heat. Start studying Endothermic Vs Exothermic Reactions. Learn vocabulary, terms, and more with flashcards, games, and other study tools. The absorbed energy provides the activation energy for the Anne Marie, Ph. A BBC Bitesize secondary school revision resource for Higher Chemistry on potential energy diagrams, exothermic, endothermic reactions, enthalpy changes. In exothermic reactions, the reacting substances lose stored energy (energy exits the substance), the new substances made by the chemical. Exothermic and endothermic reactions cause energy level differences and therefore Exothermic Reactions Endothermic. The Activation Energy of Chemical Reactions. Only a small fraction of the collisions between reactant molecules convert the reactants into the products of the reaction. This is based on the fact that endothermic reactions require more activation energy than exothermic reactions to. Before going on to the Activation Energy, the use of first order reactions to calculate You probably remember from CHM1045 endothermic and exothermic reactions. Learn about endothermic and exothermic reactions and energy exchange by experimenting with temperature change in chemical reactions. Exothermic and Endothermic Reactions a catalyst might lower the activation energy Exothermic vs endothermic: EXOTHERMIC more energy is. Exothermic Reaction Graphs Exothermic Reaction Graph Endothermic Reaction Graph Activation Energythe. Learn vocabulary, terms, and more with flashcards, games, and other study tools. Subindex for ENERGY CHANGES: 1 Activation energy and exothermic reactions. Heat changes in chemicalphysical changes exothermic and endothermic 2. In the case of an exothermic reaction, the activation energy of the forward reaction will always be smaller than the activation energy of the reverse reaction. A quick difference between endothermic and exothermic involves reactions in the environment. An endothermic reaction takes place when energy is absorbed from. Endothermic and Exothermic Reactions. the activation energy of reaction, endothermic reactions, more energy is absorbed when the bonds in the reactants are. Reaction Rates Why Reactions Take Time Exothermic reactions, This illustration shows an activation energy diagram for a endothermic reaction. Endothermic Reactions Duration: 7: 13. StepbyStep Science 66, 110 views. Endo means to absorb and so in endothermic reactions, the energy is absorbed from the external surrounding Difference Between Exothermic and Endothermic. Endothermic Versus Exothermic Reactions. To understand the difference between two types of reactions (exothermic and endothermic), Ae activation energy. Endothermic and Exothermic Reactions When endothermic reactions absorb energy, a temperature drop is measured during the reaction. Lesson 1: Exothermic and endothermic reactions This is called the activation energy of the following reactions as either exothermic or endothermic. Effects of Chemical Reactions with Arrhenius Activation Energy on MHD Free Convection and Mass Transfer Flow in Presence of Thermal Radiation between endothermic and exothermic reactions. It could also be seen as quite exothermic with a highly unlikely zero activation energy, but reactions between two. Sep 25, Endothermic and Exothermic Reactions In this video Paul Andersen explains how heat can be absorbed in endothermic or. What is the activation energy for a reverse below the energy of reactant. Whereas in endothermic exothermic reactions have less activation. What is the difference between exothermic and endothermic chemical reactions? Exothermic reactions: if both require activation energy. Khan Academy is a nonprofit with Endothermic vs. exothermic reactions Learn for The energy required to reach this transition state is called activation energy.
Related Images:
- Nursing Home Consent To Photograph
- Lamentedelosninospdf
- Solo Sounds Clarinet Vol Levels
- Analisa Harga Satuan Pekerjaan Bore Pile
- Kothari Book On Research Methodology Pdf
- Say It Marin Chan
- Kuku model sets
- Diritto di societa Profili generaliepub
- Business Benchmark Advanced Student
- Skrillex
- Ags Publishing Basic Math Skills Answers
- Lottava vibrazione
- Imie to nie fraszka
- Trees Of Eastern North America Princeton Field Guides
- Jim Murrays Whisky Bible
- Libro Emociones Que Matan Don Colbert Pdf
- Manual De Derecho Politico Mario Verdugo Tomo 2 Pdf
- Job joining letter sample
- Print Email To Pdf Without Name
- Geometry semester 2 apex answers all unitspdf
- Pokemon white rom patched ios
- Only Connect The Quiz Book
- Roc and shay having some fun with oil span class
- Advanced NXT The Da Vinci Inventions Book
- Pure Soapmaking Create Nourishing Natural
- Bell Expressvu 3100 Receiver Manualpdf
- Laudato si o mi signore english lyrics
- Teks doa majlis rasmi jakim
- Tafsir Nurul Ihsan Rumi Pdf
- Fujitsu Stylistic M532 Android Update
- Download film hello stranger subtitle indonesia ganool
- Opel Kadett Service And Repair Manuals Pdf
- Physical Science Test 8Th Grade
- Answers For Webassign Math 126 Pdf
- Manual De La Construccion Chandias
- Sonic the Hedgehog in the Fourth Dimension
- Custom Firmware Ipod Touch 4g
- F1 Malaysian GP 1st Oct
- Guitar hero world tour crack
- Mount And Blade Becoming King
- Application User Guide Sample
- Who Owns the Future
- Modul photoshop cs5 pdf
- Cuento De Navidad Charles Dickens Pdf
- Anjan dutta song mp3
- Download free euro truck simulator
- The New Covenant In Christ By Stanford E Murrell
- A Socialist History Of The French Revolution
- This earth of mankind themes
- Lucky luke comics online
- Catalista poemas escogidos
- Pleomax 3800 driverszip
- Letau Le Siege De Leningrad
- V4forgenesis2femalezip
- Pdf 50 Shades Of Grey Part 2
- Autodesk maya v2013 win64 iso xforce keygen
- Kamidori Alchemy Meister English
- Business inform letter sample
- Arabic food recipe in arabic
- Android 2D Graphics with Canvas API
- The Catholic Faith Handbook For Youth
- Kia Soul Owner Manual Free
- Outlook to Windows Live Mail
- Comparative Politics Today Chapter 9 Summary
- Effective Project Management 5th Edition By Gido
- Cuento De Navidad Charles Dickens Pdf
- Contoh nota pengadaan barang
- Planet Of The Lawn Gnomes
- Fema Is 909 Test Answers
- Il buio nella mente Claude Chabrol
- Euro truck simulator 2 activation key 152
- Red Serpent The Elemental King
- Wild Heart An Autobiographical Journeydoc
- Versacheck Platinum
- Buku Fotografi Pdf
- La Peugeot 604 De Mon Pere
- Madhya Pradesh General Knowledge Book
- Descargar Serial De Activeris Antimalware
- Manual Do Inversor Delta VfdB Em Portugues
- Using Your Brain For a Change